VSAQ for Ch 1 Chemical Reactions and Equations Class 10 Science NCERT
Important Questions1
Name and state the law which is kept in mind while we balance a chemical equation.
Answer
Law of conservation of mass.
Mass can neither be created nor can it be destroyed during a chemical reaction.
VSAQ
2
What happens when quick lime is added to water?
Answer
Quick lime reacts with water vigorously to produce slaked lime and a large amount of heat.
![]()
VSAQ
3
Which one is a chemical change–rusting of iron or melting of iron?
Answer
Rusting of iron.
VSAQ
4
State one basic difference between a physical change and a chemical change.
Answer
In a physical change, no new substance is formed. In a chemical change, new substance is formed.
VSAQ
5
Write the chemical equation for the reaction that takes place when lead nitrate and potassium iodide solutions are mixed.
Answer

VSAQ
6
Write a balanced chemical equation:
![]()
Answer
Balanced Equation:
![]()
VSAQ
7
What happens when ZnCO3 is heated in the absence of air? Give the relevant equation.
Answer
In the absence of air, ZnO(s) and CO2(g) are formed.
Chemical Equation:
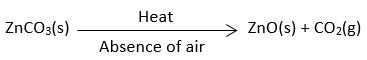
VSAQ
8
Is burning of a candle, a physical change or a chemical change?
Answer
Burning of candle is both physical and chemical change. Burning of candle melts the wax and hence physical state of wax has changed from solid to liquid. Again the wax combines with the atmosphere oxygen and changes to carbon dioxide, heat and light. Thus both the changes are accompanied in the burning of candle.
VSAQ
9
Give reason why do chips manufacturers usually flush bags of chips with gas such as nitrogen?
Answer
To prevent the oil and fats of the chips from being oxidized or become rancid.
VSAQ
10
Why is hydrogen peroxide kept in coloured bottles?
Answer
Hydrogen peroxide decomposes into H2O and O2 in the presence of sunlight and hence to prevent decomposition, they are kept in coloured bottles.
![]()
VSAQ
11
N2 + 3H2 → 2NH3, name the type of reaction.
Answer
It is a combination reaction.
VSAQ
12
Why are decomposing reactions called the opposite of combination reactions?
Answer
In combination reactions, two substances combine to form one compound and in decomposition reactions, a compound breaks down into two or more substances, so they are opposite to each other.
VSAQ
13
Why is photosynthesis considered an endothermic reaction?
Answer
Because heat is absorbed in this process.
VSAQ
14
Why do silver articles become black after sometime, when exposed to air?
Answer
They get tarnished by reacting with atmospheric air to form silver sulphide.
VSAQ
15
Write a chemical equation for double displacement reaction.
Answer
Double displacement Reaction:
Na2SO4 (aq) + BaCl2 (aq) → BaSO4(s) + 2NaCl (aq)
VSAQ
16
Write a balanced chemical equation for a chemical combination reaction.
Answer
VSAQ