NCERT Revision Notes for Surface Chemistry Class 12 Chemistry
CBSE NCERT Revision Notes1
Answer
When a solid is brought in contact with a gas or a solution its surface tends to attract and retains the molecules of the gas or the solute, e.g., when finely divided active charcoal or clay is stirred with a dilute solution of a dye, the intensity of the colour of the solution decreases. This phenomenon of attracting and retaining the molecules of a substance by a solid (or a liquid) on its surface resulting into a higher concentration of the molecules on the surface is known as adsorption.2
Answer
Adsorbent : The solid substance on the surface of which adsorption takes place is called adsorbent. Examples of absorbents are activated charcoal, Pt, Pd, Ni etc.Adsorbate : The substances, gases or liquids, which are adsorbed on the surface of adsorbent are called adsorbate.
Sorption : It may be defined as the process in which both adsorption and absorption take place simultaneously.
Desorption is process of removing an adsorbed substance from a surface on which it is adsorbed.
Absorption is different from adsorption. In absorption, the substance is uniformly distributed throughout the body of a solid or a liquid.
3
Answer
Physical adsorptionWhen the adsorbate is held on the surface by weak van der Waal’s forces, the process is called physical adsorption or physisorption.
This type of adsorption can be reversed by heating or decreasing the pressure.
Characteristics of physisorption
(a) Adsorbent has no specific preference for adsorbate i.e., it is non – specific
(b) Nature of adsorbate : More liquefiable gases are readily adsorbed.
(c) Physisorption of a gas by a solid is a reversible process.
(d) Amount of adsorption increases with increase in pressure. It involves formation of multilayers on adsorbent surface under high pressure.
(e) It is an exothermic process. Its enthalpy of adsorption is low (−20 to −40 kJ/mol−1)
Increase in temperature decreases amount of adsorption
(f) Adsorption increases with increase in surface area of adsorbent.
Chemical adsorption
When the forces holding the adsorbate on the surface are of the magnitude of chemical bond forces, the process is called chemical adsorption of chemisorption. This type of adsorption is irreversible.
Characteristics of chemisorption
(a) It is specific i.e., each adsorbent has preference for an adsorbate with which it can form chemical bonds.
(b) Chemisorption is irreversible process
(c) It has high enthalpy of adsorption (80 to 240 kJ/mol)
(d) It is an exothermic process. Rate of adsorption decreases with rise in temperature.
(e) Pressure has generally no effect on chemisorption though at very high pressure rate of adsorption increases.
4
Answer
(a) Nature of adsorbate : Readily liquefiable gases such as HCl, SO2, CO2, CH4, NH3 etc. are more easily adsorbed by the adsorbent than the permanent gases H2, N2 etc.(b) Nature of absorbent : Activated charcoal is a better adsorbent than transitional metals.
(c) Surface area of adsorbent : The greater the surface area of adsorbent, the greater will be the extent of adsorption.
(d) Pressure : Extent of physical adsorption increases as the pressure as the pressure of the gas increases, till a saturation point is reached.
(e) Temperature : Adsorption is accompanied by evolution of heat i.e., ∆H is negative, so the rate of adsorption should decrease with rise in temperature. It is found to be so in case of physical adsorption. The effect of temperature is represented by an adsorption isobar.
(f) Activation of adsorbent : An adsorbent can be activated either by heating or by bringing it in finely divided state, or by making its surface rough by rubbing. For example charcoal is activated by heating it is vacuum at 1000°C.
5
Answer
We have studied that the phenomenon adsorption arises because of attraction between the particles or molecules of adsorbent and adsorbate. Therefore, it is exothermic in nature and is accompanied by release in energy known as enthalpy of adsorption (heat of adsorption). It may be defined as :The change in enthalpy taking place for the adsorption of one mole of the adsorbate on the surface of the adsorbent.
The adsorption is also likely to influence the randomness, (T∆S) factor. The randomness of the adsorbed species on the surface of the adsorbent is likely to decrease i.e. T∆S is negative. Let us study the feasibility of the phenomenon in the light of Gibb’s Helmholtz equation. Since ∆H favours while T∆S opposes the phenomenon of adsorption, the magnitude of the former must be more. This is
feasible only at low temperature and this is what we actually notice. We shall discuss the effect of temperature on the rate of adsorption at a later stage.
6
Answer
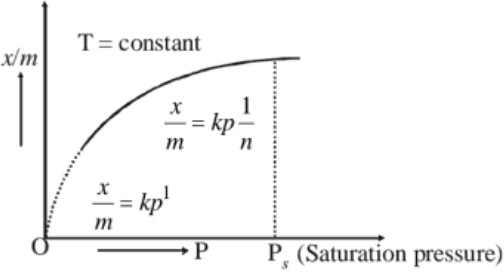
The extent of adsorption of a gas on a solid is generally expressed as x/m, where m is the mass of the adsorbent and x is the number of moles of the adsorbate at equilibrium. A relation or a graph between the magnitude of adsorption x/m and the pressure P of the gas at a constant temperature is called adsorption isotherm.Freundlich adsorption isotherm : The adsorption of a gas on a solid surface increases with increase in pressure. The variation of adsorption with pressure at constant temperature can be represented by the equation,
x/m = kp1/n
Where x = mass of adsorbate, m = mass of adsorbent, p = pressure and n are constants and depend upon the nature of adsorbate as well as adsorbent.
(a) At low pressure : x/m is directly proportional to the pressure i.e., x/m ∝ P1
(b) At high pressure : ‘The extent of adsorption, x/m becomes independent of pressure i.e., x/m ∝ P0
(c) At intermediate pressure : x/m will depend upon the power of pressure which lies between 0 and 1 i.e., x/m ∝ P1/n where n > 1 or x/m = kP1/n ….(i)
Verification of freundich adsorption isotherm equation :
On taking logarithm on both sides of (i) we get
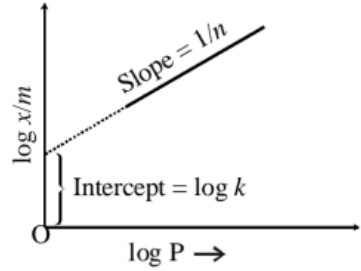
log x/m = log k + (1/n)log P ……….(ii)
Expression (i) and (ii) are called Freundlich adsorption isotherm.
Expression (ii) represents a straight line. The slope of the line will be 1/n while its intercept on the log x/m axis will be log k.
7
Answer
A graph between the amount of adsorbate adsorbed per gram of adsorbent and the temperature (T) at a constant pressure is called adsorption isobar.
8
Answer
Solid surfaces can also adsorb solutes from solutions. Fox ex :(a) When litmus solution is shaken with charcoal, it becomes colourless because the dye of the litmus solution is adsorbed by charcoal.
(b) When colourless Hg(OH)2 is precipitated in the presence of magnese on reagent, it acquires blue colour because the dye is adsorbed on the solid precipitate.
Factors affecting adsorption from solutions :
(a) Nature of the adsorbate and the adsorbent
(b) Temperature : Image
(c) Surface area : Adsorption ∝ Surface area
(d) Concentration of the solute in the solution x/m = kc1/n
9
Answer
(a) Production of high vacuum(b) Gas masks
(c) Humidity control
(d) Removal of colouring matter from solution
(e) Heterogeneous catalysis
(f) Separation of inert gases
(g) Softening of hard water
(h) De – ionization of water
(i) In curing diseases
(j) Cleaning agents
(k) Adsorption indicators
(l) Chromatographic analysis
(m) Froth floatation process for the concentration of sulphide ores.
10
Answer
The phenomenon is which a foreign substance alters the rate of reaction is called catalysisCatalyst
A substance that can influence the rate of a chemical reaction but itself remaining unchanged chemically at the end is called a catalyst.
Functions of Catalyst
(a) The main function of the catalyst is to lower the activation energy of a reaction. It is achieved by providing an alternate and easier pathway from reactants to products.
(b) In a reversible reaction, a catalyst speeds up both the forward and the reverse reactions to the same extent. A catalyst, therefore, does not change the value of the equilibrium constant.
(c) A catalyst is specific in its action, i.e., a substance that catalyses one reaction may have no effect on another similar reaction.
Types of Catalyst
(a) Positive catalyst : The substance which increase the rate of a reaction is known as a positive catalyst. It decreases the energy of activation for the reaction. For example :
(b) Negative catalyst : The substance which decreases the rate of chemical reaction is called negative catalyst or inhibitor. It increases the activation energy for the reaction. For example :
Types of Catalysis
Based on physical state
In a homogeneous catalysis, the catalyst is present in the same phase as the reactants, e.g.,
In heterogeneous catalysis, the catalyst is present in a different phase than that of the reactants, e.g.,
All the catalysts are in solid phase
11
Answer
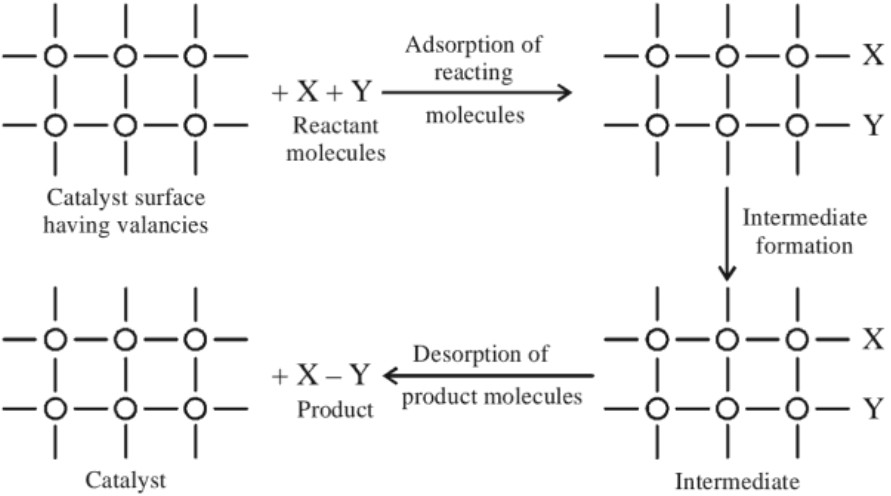
This theory explains the mechanism of heterogeneous catalysis. Modern adsorption theory is the combination of old adsorption theory and intermediate compound formation. It includes five steps :(a) Diffusion of reactant molecules to the surface of the catalyst
(b) Adsorption of reactant molecules on the surface of the catalyst.
(c) Occurance of chemical reaction on catalyst’s surface through an intermediate formation.
(d) Desorption of product molecules from the catalyst surface, which makes the surface available for more reaction to occur.
(e) Diffusion of products away from the surface of the catalyst .
12
Answer
Those substance which do not themselves act as catalysts but their presence increase the activity of a catalyst are called catalytic promoters or catalyst for catalysis. Example :In the Haber process for the synthesis of ammonia, Fe is the catalyst while molybdenum (Mo) acts as a promoter.
13
Answer
The substance whose presence decreases or destroys the activity of a catalyst is called catalytic poison. For example :- Carbon monoxide or H2S in hydrogen gas acts as a poison for Fe catalyst in the Haber process for NH3. While As2O3 acts as poison for Pt asbestos in Contact process for H2SO4 .14
Answer
Those substances which retard rate of a chemical reaction are known as inhibitors. For example : H3PO4, glycerol or acetamide decrease the rate of decomposition of hydrogen peroxide.15
Answer
It is a catalytic reaction which depends upon the pore structure of the catalyst and the size of the molecules of reactants and products, e.g., zeolites. It is microporous solid having cavities on the surface like honey comb. It can absorb a reactant which fits into its cavities zeolites are used in petrochemical industries for cracking.16
Answer
(a) Activity : It is the ability of the catalyst to accelerate the chemical reaction in case of chemisorption. No doubt, the reactants must get adsorbed reasonably strongly on the surface of catalyst to become active. However, they must not be adsorbed to strongly that may become immobile.(b) Selectivity of catalyst : The action of catalyst is quite selective in the sense that a particular catalyst may catalyse a specific reaction. Moreover, the nature of the product depends upon the nature of the catalyst.
(c) Autocatalysis : In certain reactions, one of the product acts as the catalyst. Actually in the initial stage, the reaction is quite slow. As soon as the products come into existence, one of them catalyse the reaction and increases its speed considerably.
(d) Induced catalysis : A substance that induces a similar reaction which is not otherwise possible, is called induced catalyst and this process is know as induced catalysis.
17
Answer
Enzymes are complex nitrogeneous compounds which are produced by living plants and animals. They are also called biological catalysts and are proteins which catalyse the reactions in living systems.Mechanism of enzyme catalysis
Lock and key theory :
The specific action of an enzyme with a single substrate can be explained using a Lock and Key model first postulated in 1894 by Emil Fischer. In this model, the lock is the enzyme and the key is the substrate. Only the correctly sized key (substrate) fits into the key hole (active site) on the lock (enzyme).
Smaller keys, larger keys, or incorrectly positioned teeth on keys (incorrectly shaped or sized substrate molecule) do not fit into the lock (enzyme). Only the correctly shaped key opens a particular lock. This is illustrated in the figure shown below.
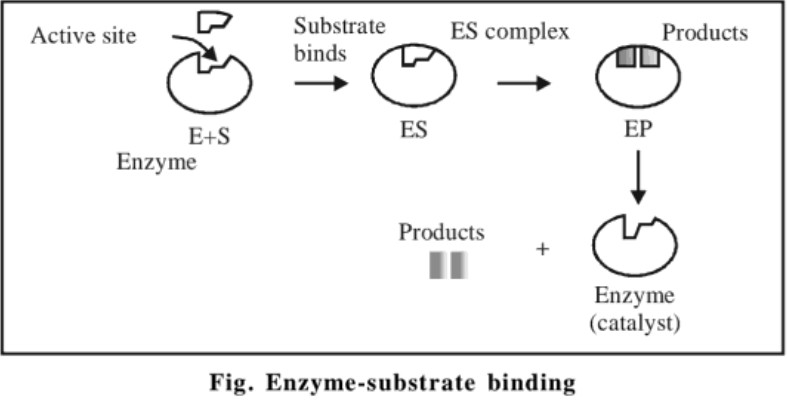
Induced fit theory
The induced – fit model assumes that the substrate plays a role in determining the final shape of the enzyme and that the enzyme is partially flexible. This explains why certain compounds can bind to the enzyme but do not react because the enzyme has been distorted too much. Other molecules may be too small to induce the proper alignment and therefore cannot react. Only the proper substrate is capable of inducing the proper alignment of the active site.
Characteristics of enzyme catalysis
Main features of enzyme catalysis are :
(a) Their efficiency is very high even at low concentration.
(b) They are highly specific, e.g., urease, an enzyme that catalysis the hydrolysis of urea.
None of the enzymes except urease, present in the cell catalysis the above reaction.
(c) They are highly sensitive to temperature changes.
(d) They are highly sensitive to acidity or alkalinity of the solution.
18
Answer
A colloid is a heterogeneous system in which one substance is dispersed a fine particles in another substance called dispersion medium. The main difference between a suspension, a colloid and true solution is their particle size. In suspension particle size is greater than 10−5 cm or 103A°. In colloids particles size range between 10−7 to 10−5 cm. While in true solution particle size is less than 10−7 cm.
Classification of Colloids
(a) Depending on the physical states of dispersed phase or dispersion medium. 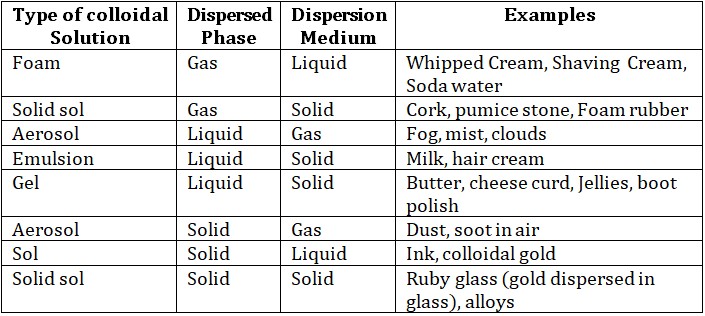
(b) Based on nature of interaction between dispersed phase and dispersion medium. 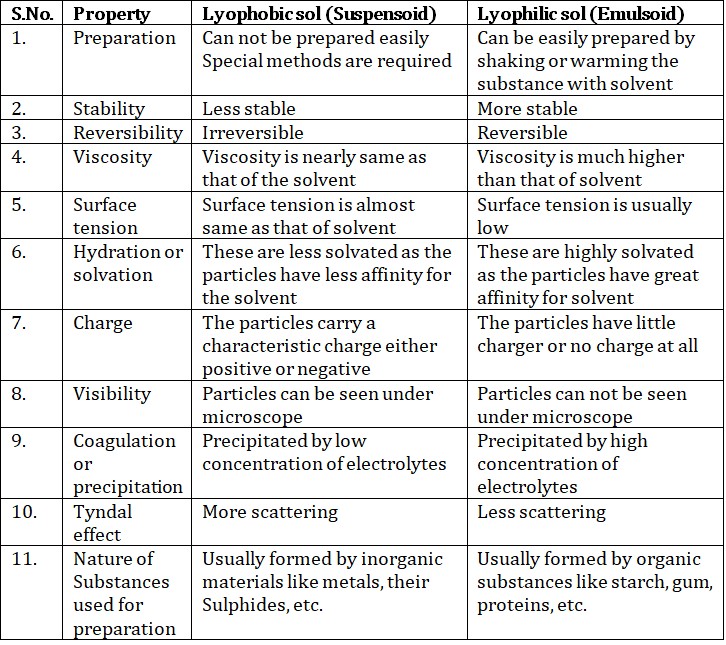
(c) Based on the size of particles of the dispersed phase :
(i) Multimolecular colloids – In this case the colloidal particles consists of aggregates of atoms or small molecules with diameters of less than 1nm eg gold sol, sulphur sol.
(ii) Macromolecular colloids – In this case dispersed particles are of colloidal size and are called macromolecules, usually polymers such as starch, cellulose, proteins or synthetic polymers etc.
(iii) Associated colloids - At low concentrations they behave as electrolytes and at higher concentrations as colloids. They are also known as micelles. Soaps and detergents are the examples. The micelles may contain about 100 molecules or more.
The formation of a micelle depends upon the concentration of the dispersed phase and also the temperature. It can occur only above a particular concentration called critical micelle concentration (CMC) and above a particular temperature known as Kraft temperature.
19
Answer
Let us explain the mechanism of micelle formation with the help of soaps. These are the sodium or potassium salts of higher fatty acids with the general formula RCOO− Na+ where R is a long chain alkyl group. These are non – polar alkyl group (R) and a polar carboxyl group (COO−). The hydrocarbon chain represented by alkyl group is quite long and is water repelling in nature due to its non – polar character whereas water molecules are polar. It is thus, hydrophobic (water repelling) and is known as the hydrophobic tail or simply tail. The carboxyl group is water loving and constitutes the head.In aqueous soap solution, the RCOO− ions are present on the surface with COO− groups pointing inwards while the alkyl or hydrocarbon chain (R) remain at the surface. However, at critical micelle concentration (C.M.C.), these anions are pulled into the bulk of the solution and get aggregated resulting in spherical shape. In the sphere, the hydrocarbon chains(tail) point towards the centre while COO− ions (head) remain outward on the surface of the sphere. The aggregate thus, formed is known as ‘ionic micelle’ or simply ‘micelle’.
20
Answer
Sols are the colloidal system in which the solid is disperse phase and the liquid is dispersion medium.Hydrosols – Colloids in water.
Alcosols – Colloids in alcohol. |
21
Answer
Colloids can be prepared by following methods :(a) Chemical methods:
(i) Oxidation , e.g., a sulphur sol is prepared by oxidation of H2S.
SO2 + 2H2S → 2H2O + 3S(sol)
(ii) Reduction : The colloidal solutions of metals like gold silver, platinum, etc., are obtained by the reduction of their dilute salt solutions by formaldehyde or hydrazine.
2AuCl3 + 3HCHO + 3H2O → 2Au (sol) + 3HCOOH + 6HCl
PtCl4 + 2HCHO + 2H2O → Pt(sol) + 2HCOOH + 4HCl
(iii) Hydrolysis : The sols of hydrated oxides of weakly electropositive metals like Fe, Al, Sn, etc., are prepared by this method. Fe(OH3) sol is obtained by pouring a dilute solution of FeCl3 into boiling water.
FeCl3 + 3H2O → Fe(OH)3 + 3HCl
(iv) Double decomposition : This method involves preparation of colloids from inorganic salts e.g., As2O3 + 3H2S → As2S3 (sol) + 3H2O
(b) Condensation methods:
(i) Exchange of solvent : A colloidal sol is obtained when a true solution is mixed with an excess of another solvent in which the solute does not dissolve but the solvent dissolves, e.g., sulphur sol is obtained when solution of sulphur in alcohol is mixed with excess of wate.
(ii) Excessive cooling : A colloidal sol of ice in an organic solvent (CHCl3 or ether) can be obtained by freezing a solution of water in the solvent.
(iii) Peptization method : The process of transferring a freshly precipitated substance into a colloidal state is called peptization. This is done by the addition of a small amount of a suitable electrolyte to a freshly precipitated substance. Thus, a sol of ferric hydroxide is obtained by treating fresh precipitate of Fe(OH)3 with dilute FeCl3 solution.
(c) Dispersion methods:
(i) Mechanical dispersion : In this method, the course suspension of the substance is brought into the colloidal state in the dispersion medium by grinding it in a colloidal mill.
(ii) Bredig’s arc method: When an electric arc is struck between two electrodes of a metal (Au, Ag, Pt, Cu, etc.) in water containing traces of an alkali, the metal passes into the colloidal solution.
Note : Lyophilic sols are readily prepared by warming the substance with a dispersion medium, e.g., starch, gelatin, gum Arabic, etc., are easily brought into the colloidal state by warming with water.
22
Answer
(a) Dialysis : The process of separating a soluble crystalloid from a colloid is called dialysis. The purification of a colloid by dialysis is based on the fact that colloidal size particles cannot pass through parchment paper or cellophone membrane, whereas small molecules or ions can pass thought it. Thus, when a bag of parchment paper containing a colloidal solution is suspended into pure water, the small molecules and ions pass through the membrane into the pure water and the pure colloidal solution is left behind.(b) Electrodialysis : Dialysis in presence of applied electric field is called electro – dialysis. In this process; electric field is applied by dipping two electrodes in the container. Electrodes attract ions of opposite charge and speed up the process of purification.
(c) Ultrafiltration : Sol particles directly pass through ordinary filter paper because their pores are larger (more than 1μ or 1000mμ) than the size of sol particles (less than 200 mμ). But if the pores of the ordinary filter paper are made smaller by soaking the filter paper in a solution of gelatin or collodion and subsequently hardened by soaking in formaldehyde, the treated filter paper may retain colloidal particles and allows the true solution particles to escape. Such filter is known as ultrafilter and the process of separating colloids by using ultrafilters is known as ultrafiltration.
23
Answer
(a) Colour – The colour of hydrophobic sol depends on the wavelength of the light scattered by the dispersed particles. The wavelength of the scattered light again depends on the size and the nature of particles. Particles of same size scatter light of same wavelength and have same colour.(b) Colligative properties – These properties depend on the number of solute particles in solution. In case of colloidal solutions., colloidal particles are the aggregates of many ions or smaller molecules and when compared to true solutions or normal solutions, the total no. of particles of solute in solution are very less and hence these solutions exhibit colligative properties to lesser extent.
(c) Optical properties – sols exhibit Tyndall effect
When a beam of light is passed through a sol and viewed at right angles, the path of the light shows up as a hazy beam of cone (laminated path by bluish light). This was first observed by Faraday and later by Tyndall and is known as Tyndall effect. The bright cone of the light is called ‘Tyndall cone’. The Tyndall effect is due to the fact that the colloidal particles absorb light and scatter in all directions in space. The scattering of light illuminates the path of the beam in the colloidal dispersion. Thus, Tyndall effect is defined as the scattering of light by the colloidal particles present in a colloidal sol.
(d) Kinetic properties – When a sol is examined with an ultramicroscope, the suspended particles are seen as shining speeks of light. By following an individual particle, it is observed that the particle is in a state of continuous motion in zig – zag directions. The continuous rapid zig – zag motion of a colloidal particle in the dispersion medium is called Brownian movement or motion (first observed by British botanist Robert Brown). The Brownian movement arises due to the unbalanced bombardments of the particle by the molecules of dispersion medium
(e) Charge on colloidal particles – Colloidal particles always carry an electric charge. The mutual forces of repulsion between similarly charged particles prevent them from aggregating and settling under the action of gravity. All the dispersed particles in a colloidal solution carry the same charge while the dispersion medium has an equal and opposite charge.
Electrophoresis – If electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode, due to charge on them. The movement of sol particles under an applied electric potential is called “Electrophoresis”. Depending upon the direction of movement of particles towards cathode or anode, electrophoresis can be called “cataphoresis’ or ‘Anaphoresis’.
Electro – osmosis – The medium will move in opposite direction to the dispersed phase under the influence of applied electric potential. The movement of dispersion medium under the influence of applied potential is known as ‘Electro – osmosis’.
24
Answer
By addition of electrolytes : When an electrolyte is added in excess to a sol, then the electrolyte furnishes both type of ions in solution. The oppositely charged ions get adsorbed on the surface of colloidal particles which causes neutralization and thus the size and mass of colloidal particle increases and it becomes a suspension particle. Due to greater volume and greater mass these suspension particles settle down i.e. they coagulate.Hardy Schulze rule – “It states that the precipitating effect of an ion on dispersed phase of opposite charge increases with the valence of the ion.”
The higher the valency of the flocculating ion, the greater is its precipitating power. The minimum concentration of an electrolyte which is required to cause the coagulation of a sol in two hours is known as flocculation value. Few other methods for coagulation
(a) By persistent dialysis
(b) By elelctrophoresis.
(c) By mixing two oppositely charged sols.
(d) By boiling.
25
Answer
The property of lyophilic sols to prevent the precipitation or coagulation of a lyophobic sol is called protection.Protective Colloid
The Lyophilic sol used to protect a lyophobic sol from precipitation is referred to as a protective colloid. Lyophilic sols form a thin layer around lyophobic sol or around the ions furnished by electrolyte and therefore the coagulation can not take place.
Gold number
The protective power of lyophilic colloids is measured in terms of ‘Gold number’ which is defined as “The number of milligrams of a hydrophilic colloid that will just prevent the precipitation of 10 ml of gold sol on addition of 1 ml of 10% NaCl solution is known as gold number of that protector (Lyophilic colloid)”
The smaller the gold number of a protective lyophilic colloid, greater is its protection power.
26
Answer
It is colloidal system in which both the dispersed phase and the dispersion medium are liquids, e.g., milk consists of small drops of liquid fat dispersed in water.Type of Emulsions
(a) Oil – in – water type in which small droplets of an oil are dispersed in water, e.g., milk, cod – liver oil, etc.
(b) Water – in – oil type in which water droplets are dispersed in an oil medium, e.g., butter.
Preparation of Emulsions
Emulsions are prepared by vigorously agitating a mixture of two relevant liquids. Droplets of one liquid undergo dispersion in other liquid. The emulsions so prepared are never stable. On keeping, they separate out into two distinct layers. The emulsions are stabilized by adding a substances known as emulsifying agent, e.g., soaps, detergents, proteins, gum and agar.
Properties of Emulsions
Emulsions can be broken into constituent liquids by heating, freezing, centrifuging or by adding electrolyte etc. The droplets of dispersed phase of emulsion are often negatively charged.
Emulsions show Brownian movement and Tyndall effect.
27
Answer
(a) Blood: Blood acts as a colloid as it has albuminoid suspended in water. Alum and FeCl3 solution stop bleeding due to coagulation.
(b) Food : Ice cream, butter, milk, cream etc. are colloids.
(c) Blue colour of sky : Dust particles and water vapours dispersed in air act as a colloid. They scatter the blue light which makes the sky looks blue. (Tyndall effect)
(d) Delta formation : River water is a colloidal solution of clay and sea water contains a number of electrolyte. Hence when sea water comes in contact with river water, the colloid present in river water undergo coagulation due to the action of electrolyte. These colloids are deposited at the meeting point of water forming a delta.
(e) Rain: Clouds are aerosols in which small droplets are suspended in air. On condensation these droplets combine to form bigger drops and finally fall down as rain. Rainfall takes place when two oppositely charged clouds meet each other. It is possible to cause artificial rain by throwing electrified sand on clouds from an aeroplane.
(f) Soils : Soils are colloidal in nature. Soil absorbs moisture and other nourishing materials due to its colloidal nature. Humus acts as protective colloid.
Application of Colloids
The important applications of colloids are :
- Precipitation of solid particles from smoke coming from chimney of industries.
- Purification of drinking water by coagulating the suspended impurities with the help of alum.
- Most of the medicines are prepared in colloidal form.
- Tanning of leather is based on the principle of coagulation of positively charged hide using negatively charged tannin.
- Cleansing action of soaps and detergents are due to micelle formation.
- Photographic plates and films contain coating of an emulsion of the light sensitive silver bromide in gelatin.
- Natural rubber is a colloid of latex in water.
Paints, inks, synthetic plastics, rubber, graphite lubricants, cement etc. are all colloidal solutions.