SAQ for Chapter 5 Changes Around Us: Physical and Chemical Class 7 Science NCERT
Important Questions1
Q1: Explain physical reaction along with examples.
Answer
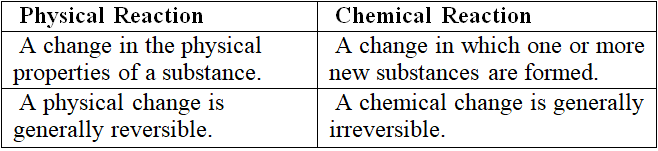
A change in which a substance undergoes a change in its physical properties is called a physical change. For example; melting of ice.
SAQ
2
Q2: Explain chemical reaction along with examples.
Answer
A change in which one or more new substances are formed is called a chemical reaction. For example; rusting of iron.
SAQ
3
Q3: Differentiate between physical and chemical reactions.
Answer
SAQ
4
Q4: State the condition necessary for the occurrence of any reactant.
Answer
For the occurrence of any reaction, the following condition is necessary:• The molecules or atoms of the reactants must collide with each other.
• This collision is essential to break old bonds and form new ones.
SAQ
5
Q5: Why new products are formed in a chemical reaction?
Answer
New products are formed in a chemical reaction due to the following reasons:• The old bonds between atoms in the reactants are broken.
• New bonds are created, leading to the formation of different substances.
• The resulting products have different properties compared to the original reactants.
SAQ
6
Q6: Explain the process of rusting.
Answer
The process of rusting occurs when iron is exposed to damp air or water for a long time. This results in a reddish-brown flaky substance known as rust. The key steps in rusting are:• Iron reacts with oxygen from the air.
• This reaction requires the presence of water or moisture.
• The outcome of this reaction is a compound called iron oxide.
Rusting is accelerated in humid conditions, making it a significant issue for iron objects.
SAQ
7
Q7: How can we prevent rusting?
Answer
o prevent rusting, it is essential to keep iron, water, and oxygen apart. Here are some effective methods:• Apply a coat of paint or grease regularly to create a barrier.
• Use wax or oil coatings, especially for vehicles.
• Keep tools and equipment dry; wipe them down after use.
• Store items in a dry place to avoid moisture.

SAQ
8
Q8: Setting of curd is regarded as a chemical change, explain why?
Answer
The setting of curd is considered a chemical change for the following reasons:• The original substance, milk, cannot be recovered after curd is formed.
• Curd has distinct taste, smell, and chemical properties compared to milk.
• New substances are created during the process, indicating a transformation.
SAQ
9
Q9: What happens when baking soda is treated with vinegar?
Answer
When baking soda is mixed with vinegar, a reaction occurs that produces a hissing sound and bubbles. This is due to the formation of carbon dioxide gas. The chemical equation for this reaction is:• Vinegar (Acetic acid) + Baking soda (Sodium hydrogen carbonate) → Carbon dioxide + other substances.
The bubbles you see are the carbon dioxide gas being released during the reaction.
SAQ
10
Q10: What happens when a piece of iron metal is placed in copper sulphate solution?
Answer
When iron is placed in Copper sulphate solution, the iron is coated with a brown coloured substance "copper" and CuSO4 solution changes from blue to light green. This is due to iron displacing copper as it is more reactive than copper.
SAQ
11
Q11: Explain the changes occuring in burning of candle.
Answer
When a candle burns, it undergoes both physical and chemical changes:• The wax melts as it heats up, which is a physical change because it can solidify again when cooled.
• As the candle burns, it produces light and gases, such as carbon dioxide, indicating a chemical change due to the combustion of the wick.

SAQ
12
Q12: Baking soda is mixed with lemon juice, bubbles are formed with the evolution of gas, explain the changes occurred here.
Answer
When baking soda is mixed with lemon juice, a chemical reaction occurs, resulting in the formation of bubbles. This happens because:• A new substance, carbon dioxide, is produced during the reaction.
• The release of gas creates visible bubbles.
• This process is an example of a chemical change as new substances are formed.
SAQ
13
Q13: Name some of the process in which both chemical and physical changes take place.
Answer
One example of a process that involves both chemical and physical changes is the burning of wood:• The moisture in the wood turns to vapor when heated, which is a physical change.
• The actual burning produces carbon dioxide and other products, indicating a chemical change.
SAQ
14
Q14: Explain why burning of wood and cutting it into small pieces are considered as two different types of changes.
Answer
Burning of wood produces ash and smoke. Hence the properties of wood are changed and new substances are formed.
So, it is a chemical reaction. When a log of wood is cut into small pieces,there is no new substance formed. Each small piece bears the properties of wood. So, its a physical change.Obviously, burning and cutting of wood are two different types of changes.

SAQ
15
Q15: Explain how painting of an iron rod prevents it from rusting.
Answer
To prevent rusting, it is essential to stop iron from contacting both air and moisture. Painting an iron gate achieves this by:• Creating a protective barrier that blocks air and moisture.
• Reducing the chances of rust formation.
• Ensuring the iron remains dry and free from corrosive elements.
Regular maintenance of the paint layer is important to keep the iron protected.
SAQ
16
Q16: A part from new products, many other things accompany a chemical change, what are those things?
Answer
Part from new products, many other things accompany a chemical change, those things are:• Heat, light or any other radiation may be given off or absorbed.
• Change in smell may take place.
• Sound may be produced.
• Change in colour may take place.
• A gas may be formed
SAQ
17
Q17: Burning of any substance is the chemical change. Discuss.
Answer
Burning of any substance is a chemical change. Burning involves a series of changes:• Heat is always produced.
• The old bonds in the reactants are broken.
• New bonds are formed, leading to new products.
• The properties of these new products differ from the original substances.
Thus, burning is a clear example of a chemical change.
SAQ
18
Q18: State Four characteristics which are included in the physical properties of matter.
Answer
Physical properties of matter include the following characteristics:• Shape: The form or outline of an object.
• Size: The dimensions or magnitude of an object.
• Colour: The visual perception of light reflected from an object.
• State: The physical form of matter, such as solid, liquid, or gas.
SAQ
19
Q19: Why formation of manure from leaves is a chemical change?
Answer
The formation of manure from leaves is a chemical change because:• It produces a new substance with a different composition.
• The original leaves undergo a transformation that alters their chemical structure.

SAQ
20
Q20: Why cutting of wood is a physical change?
Answer
Cutting of wood is classified as a physical change for the following reasons:
• The identity of the wood remains the same.
• Its composition does not change.
• No new substances are formed during the process.
Thus, cutting wood is a reversible change that affects its shape and sizebut not its fundamental properties.
SAQ