Summary for Heat Transfer in Nature Class 7 science
Book Solutions1
Answer
Pema and her brother Palden live in Gangtok. One cold winter evening, they sit near a fireplace. Palden talks about his trip to Kerala during winter. He says Kerala is warmer and more humid than Gangtok.
Their grandfather explains, “Kerala is closer to the equator and has a long coastline, so it is warmer and more humid.” Palden adds, “We learned that the Sun is the main source of heat and light, and places near the equator are usually hot.”
While they talk, Pema watches her grandmother cooking thukpa (a traditional Sikkimese dish) in a big metal pan. She asks, “Why are cooking utensils usually made of metal?” Palden answers, “Because metals are good conductors of heat,” which they studied in their science chapter.
Let’s dive into nature of heat and its transfer in nature!
2
Answer
Conduction is the process by which heat is transferred from the hotter part of an object to its colder part through direct contact. In solids, especially metals, particles vibrate when heated and pass this energy to neighboring particles without moving from their positions. This makes conduction an important process in everyday activities like cooking.
How Conduction Works: When one end of a metal object is heated, the particles at that end gain energy and vibrate more. These vibrations are passed to adjacent particles, transferring heat along the object. For example, when a metal pan is heated, the heat travels from the flame to the entire pan, making it hot.
Let us perform an activity to learn why certain materials are good conductors of heat.
Materials Needed: Metal strip (aluminium or iron), Four pins, Candle or spirit lamp, Stand (or two bricks for support), Wax to attach the pins, Heat source
Steps:
• Take a metal strip (15 cm long) and attach four pins to it using wax, spaced about 2 cm apart.
• Secure the strip to a stand or between two bricks.
• Heat the end of the strip away from the stand with a candle or spirit lamp.
• Observe and predict the behaviour of the pins.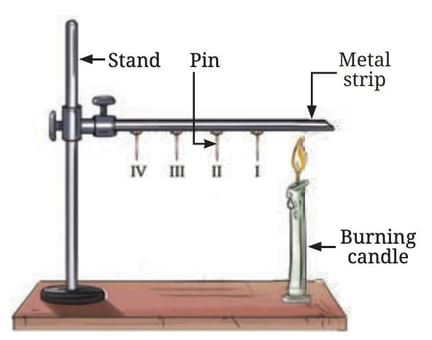
Heat transfer in a metal strip
Prediction: You are asked to predict the order in which the pins will fall as the strip is heated.
Observation: The first pin (Pin I), closest to the candle flame, falls first, followed by the other pins in order (II, III, and IV). The reason for the sequential fall of the pins is the process of heat conduction.
Conclusion
• Conduction is the process of heat transfer from the hotter part of a material to the cooler part. As the heat travels along the metal strip, it causes the wax holding each pin to melt, leading to the pin falling.
• The heat is transferred from the hot end (near the flame) to the colder end through the metal particles vibrating and passing on the energy to their neighbours.
• Metals are good conductors of heat, which is why metal utensils are used for cooking.
Conductors and Insulators of Heat
1 Good Conductors of Heat:
• Metals (e.g., aluminium, iron) allow heat to pass through them easily.
• This is why metal cooking utensils are commonly used.
2 Poor Conductors of Heat (Insulators):
Materials like wood, glass, clay, and porcelain do not allow heat to pass through easily.
For example:
• Tea or coffee cups made of clay or porcelain help in retaining heat longer.
• Woollen fabrics trap air, which is a poor conductor, and help keep us warm.
• The presence of air between layers of clothing (such as woolen clothes or blankets) reduces heat flow and helps keep us warm. 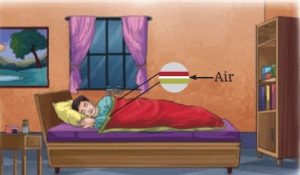
Air trapped between two thin blankets acts as an insulator
How Air Acts as an Insulator and Its Applications in Daily Life
1 Woollen Fabric and Heat Retention
• Woollen fabric traps air in its tiny pores or gaps.
• Since air is a poor conductor of heat, it reduces the flow of heat from our bodies to the cooler surroundings.
• This trapped air acts as insulation, helping to keep us warm in cold weather.
• Similarly, when multiple layers of clothing trap air between them, the air acts as an insulator and keeps the body warm.
2 Blankets and Trapped Air
• The presence of air between two thin blankets makes them warmer compared to a single thick blanket.
• This is because the air layer between blankets slows down heat loss from our body, making us feel warm and cozy.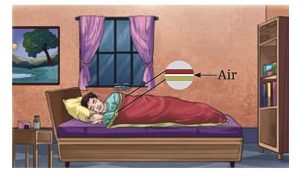
3 Insulated Houses: Using Heat Transfer Principles
• Is it possible to build houses that remain comfortable inside despite very hot or cold outside weather?
• Yes, houses in extreme climates use the concept of heat transfer to stay warm in winter and cool in summer.
4 Hollow Bricks and Heat Insulation
• Some houses use hollow bricks for their outer walls.
• Air trapped inside the hollow parts of these bricks acts as a poor conductor of heat.
• This trapped air helps keep the house warm in winters by reducing heat loss and cool in summers by reducing heat gain.
• Thus, hollow bricks help maintain a comfortable indoor temperature by slowing heat transfer through the walls.
Fascinating Facts
• The upper regions of the Himalayas, such as the Mori block of Uttarkashi in Uttarakhand, experience extremely cold climates and heavy snowfall in winters.
• To keep houses warm in such harsh conditions, people build walls with two wooden layers.
• The space between these wooden layers is filled with cow dung and mud.
• Both wood and mud are poor conductors of heat, meaning they do not allow heat to escape easily.
• This natural insulation prevents heat loss, helping keep the houses warm and cozy during winter.
3
Answer
Convection is the process of heat transfer in liquids and gases, where heated particles move and carry heat with them. This movement creates currents, such as breezes near the sea or the rising of smoke, making convection a key process in nature.
How Convection Works?
• When a liquid or gas is heated, its particles gain energy, become less dense, and rise.
• Cooler, denser particles then move to take their place, creating a cycle of movement called a convection current.
• For example, when water is heated in a pan, the warmer water rises, and cooler water sinks, distributing heat.
Convection in Gases: In air, heated air expands, becomes lighter, and rises.
For example: Smoke rises because it is made up of hot gases and tiny solid particles that are released when something burns.
When these particles are heated, they become lighter than the surrounding air, causing them to rise.
This is similar to what happens when air is heated; it expands, takes up more space, and becomes less dense, which is why warm air also rises. 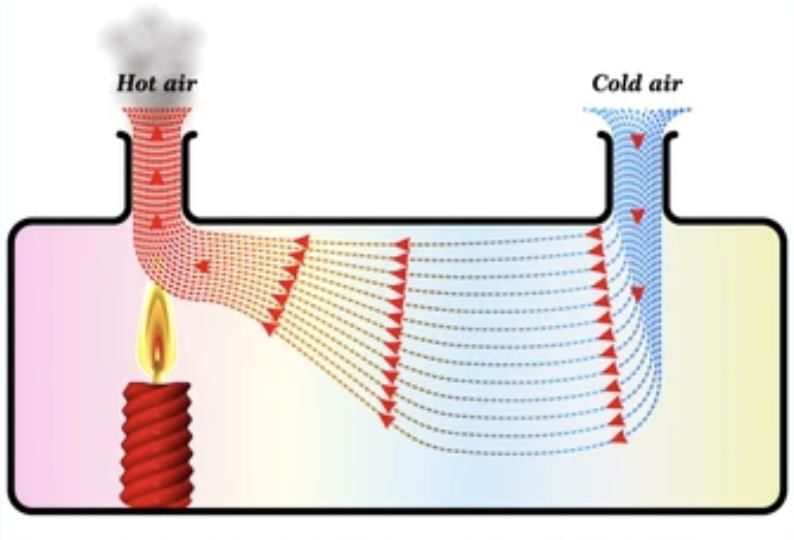
Convection in Liquids: In liquids like water, heated particles rise, and cooler ones sink, creating a circular flow. 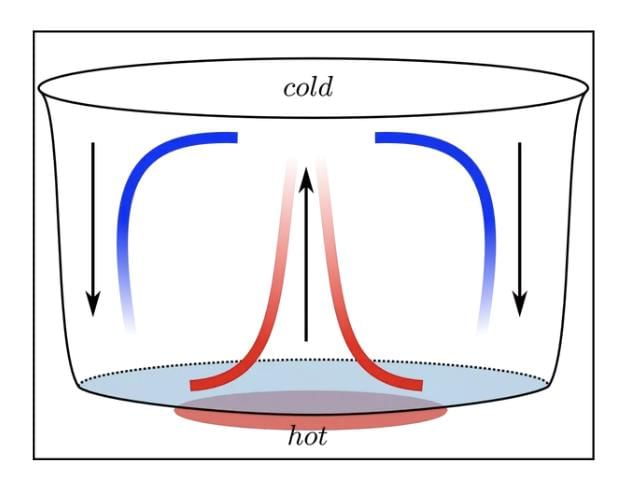
To understand why smoke rises more clearly, let us perform an activity.
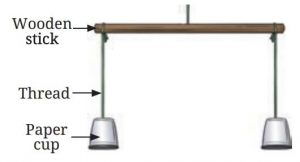
Materials Needed: paper cups, a wooden stick, threads, a burning candle.
Procedure:
• Hang the paper cups in an inverted position on the wooden stick using threads.
• Place the stick horizontally.
• Light a candle and place it under one of the cups.
• Observe what happens to the cups.
Observations and Explanation:
• The cup with the candle underneath it rises because the air inside the cup heats up.
• When air is heated, it expands and takes up more space, making it lighter. This causes the cup to rise. 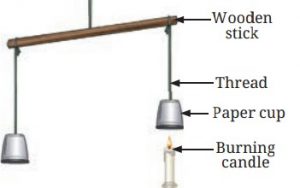
Hot air rising up
Example of Air Expansion:
• When a partially inflated balloon is placed in the Sun, the air inside it heats up and expands, causing the balloon to become larger.
Smoke Rising:
• When an incense stick is burnt, the smoke produced is a mixture of hot gases and tiny solid particles.
• Since the smoke is warmer than the surrounding air, it rises.
Convection in Liquids:
To understand how heat transfer occurs in liquids, we can perform an activity.
Materials Needed: 500 mL beaker, water, straw, potassium permanganate, candle.
Procedure:
• Fill the beaker halfway with water.
• Using a straw, place a grain of potassium permanganate at the center of the beaker’s base.
• Place a candle under the center of the beaker’s base.
• Observe the movement of the colored streak in the water.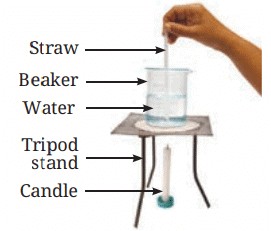
Observations:
• As heat is supplied, a streak of color starts moving up from the center and coming down from the sides of the beaker. 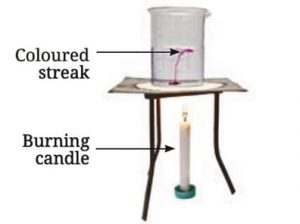
Demonstration of convection in heated water
Explanation:
• The water at the bottom of the beaker gets heated, becomes lighter due to expansion, and rises.
• The cooler, heavier water from the sides then comes down to take its place.
• This creates a continuous cycle until the entire volume of water is heated.
Conclusion:
• The movement of the colored streak in the water demonstrates convection, which is how heat transfer occurs in liquids and gases through the movement of particles.
• Just like air, water gets heated through convection, where particles move from one place to another, carrying heat with them.
4
Answer
• During the day, the land near the beach heats up faster than the water in the sea. This is because different materials absorb heat at different rates.
• However, at night, the situation changes: the land cools down faster than the water.
• This difference in how quickly land and water heat up and cool down is what causes the land and sea breeze.
Let us check how land and water get heated and cooled by performing an activity.
Materials Required: Two identical bowls, Soil , Water and Two laboratory thermometers
Procedure:
1. On a clear, sunny day, under the supervision of a teacher or an adult, take two identical bowls.
2. Fill one bowl halfway with soil and the other bowl halfway with water.
3. Fix a laboratory thermometer in each bowl, ensuring that the bulbs are immersed in the soil and water, respectively, and do not touch the bottoms or sides of the bowls.
4. Place the set-up in sunlight.
5. Observe the rise in temperature of the soil and water over a period of time.
Observations:
After 20 minutes, you will find that the temperature of the soil rises more than that of the water. This indicates that the soil heats up faster than water.
Cooling Experiment:
1. After letting the soil and water heat up, bring the set-up indoors and allow it to cool for 20 minutes.
2. Observe the cooling rates of the soil and water.
Conclusion:
• Soil heats up faster than water.
• Soil also cools faster than water.
Sea Breeze
• During the day, when the land heats up quickly, the air above the land also becomes warm and rises.
• This creates a low-pressure area over the land.
• Meanwhile, the air above the sea is cooler and denser.
• To fill the low-pressure area over the land, the cooler air from the sea moves in, creating a sea breeze.
• This is why people living in coastal areas feel a refreshing breeze coming from the sea during the day.
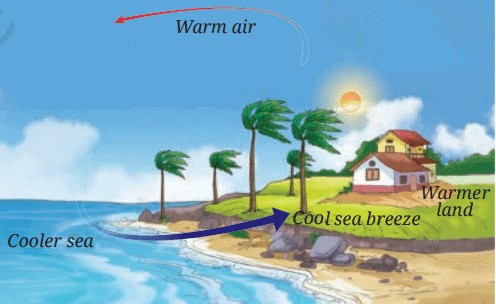
Sea Breeze
Land Breeze
• At night, the land cools down faster than the sea.
• The air above the land becomes cooler and denser, creating a high-pressure area.
• Meanwhile, the air above the sea is still relatively warm and rises, creating a low-pressure area.
• To balance the pressure, the cooler air from the land moves towards the sea, creating a land breeze.
• This is why people living near the shore experience a change in wind direction from day to night.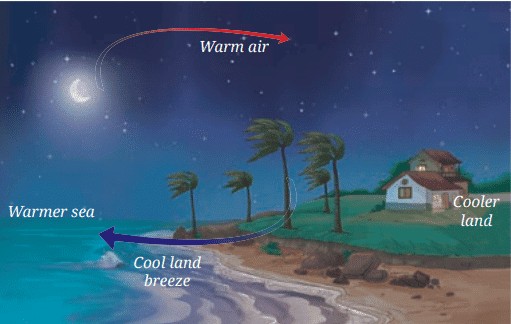
Land Breeze
5
Answer
• Radiation is heat transfer do not need any medium. All objects emit heat this way.
• Heat transfer happens directly from the a hot object to us through a process called radiation.
• For example, the Sun’s heat reaches Earth by radiation. The Sun’s hot surface (about 6000°C) emits energy waves, some of which warm the Earth.
• Radiation does not require a medium, which is why we can feel the warmth of the Sun even though space is a vacuum.

Examples of Heat Transfer in Daily Life ( Combining all the three Processes)
Many everyday examples show conduction, convection, and radiation happening at the same time.
For example, when water is heated in a pan:
• Heat moves from the flame to the pan by conduction.
• Water inside the pan heats up by convection.
• The warmth we feel around the flame and pan is due to radiation.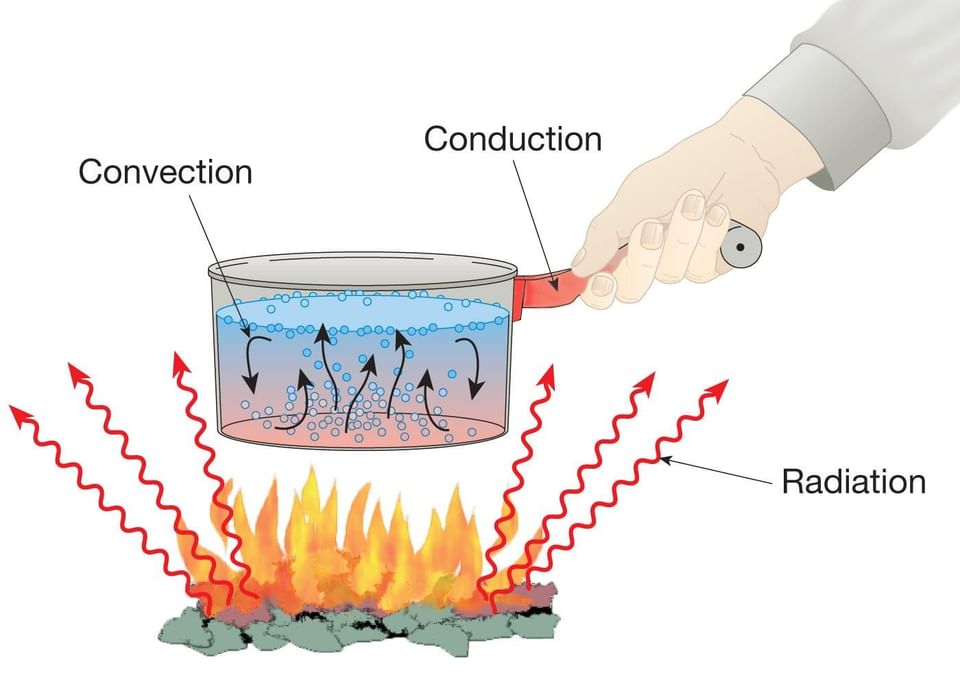
Fascinating Facts: The Himalayan Bukhari
In the upper Himalayan region, a traditional room heater called bukhari is used to keep rooms warm in winter. It is an iron stove where wood or charcoal is burned. A long pipe attached at the top acts as a chimney to release smoke. The flat top of the bukhari can also be used for cooking by placing utensils on it.
When the bukhari is used, all three types of heat transfer—conduction, convection, and radiation—work together to warm the room and cook food.
6
Answer
Water exists in three states in nature
Liquid: in oceans, rivers, lake
Solid: as snow, glaciers, ice sheets in mountains and polar regions
Gas: as water vapor in the atmosphere
• During
summer, snow and ice melt due to the Sun’s radiation, forming rivers that flow
into oceans. Fresh snow replenishes the ice in winter.
• Water
in oceans, rivers, and lakes evaporates due to the Sun’s heat. Plants also
release water vapor through transpiration.
• Water
vapor rises, cools, and condenses to form clouds. Clouds cause precipitation
(rain, snow, hail).
• This
continuous movement of water—evaporation, condensation, precipitation,
infiltration, and runoff—is called the water cycle 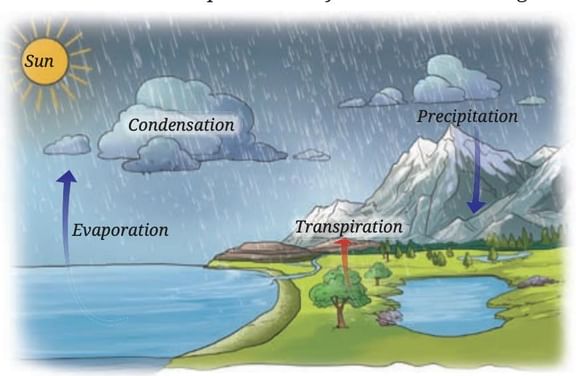
Water cycle
Importance of Water Cycle
The water cycle is the process through
which water continuously moves upward as water vapor and downward through
precipitation, passing through soil, rocks, and plants before returning to
water bodies. This cycle helps to redistribute and replenish water in rivers,
lakes, and oceans while conserving the total amount of water on Earth.
Know a Scientist: Varahamihira
Varahamihira was a famous astronomer
and mathematician of the 6th century CE from Ujjaini (now Ujjain),
Madhya Pradesh. In his work Brihatsamhita, he described methods
to predict seasonal rainfall. His predictions were based on observations of
cloud formation, wind patterns, the positions of stars and the moon, and other
natural phenomena.
Seepage
of Water Beneath the Earth's Surface
Let's Fisrt Perform Activity to
understand : How does water seep through the surface of the Earth?
• Take
three transparent, used plastic bottles of 1 L capacity.
•Cut
them in the middle and make a small hole in the cap of each bottle.
• Keep
them inverted and put some clay in one bottle, sand in the second, and gravel
in the third.
• Place
three identical beakers below each bottle.
• Add
200 mL of water to each bottle.
• Predict
the amount of water flowing out of each bottle.
• Collect
the water that flows through each bottle for 10 minutes.
• Compare
the amount of water that comes through each bottle. 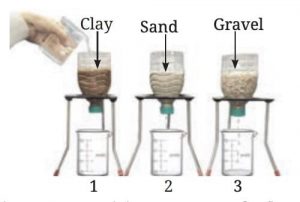
An activity to compare the flow of
water through clay, sand and gravel
You may have observed that water flows
fastest through gravel, slower through sand, and slowest through clay. This is
due to the differences in the particle sizes and the spaces between them:
• Gravel: The spaces between gravel particles are wider,
allowing water to pass through quickly.
• Sand: The particles are smaller than gravel, so the
spaces are narrower, causing slower water flow.
• Clay: The smallest particles create very tight spaces,
restricting water flow the most.
Now let's learn the theory and
definition
• When
rainwater or surface water seeps down through the soil and rocks beneath the
Earth’s surface, this process is called infiltration.
• The
ease with which water infiltrates depends on the size and connectivity of the
spaces between soil and rock particles. If these spaces (called pores) are wider,
open, and well connected, water seeps through more quickly and easily.
• Once
water infiltrates, it moves down and gets stored in the tiny spaces or pores
within sediments (loose soil, sand, gravel) and the cracks or openings in rocks
below the surface. This stored water is known as groundwater.
• The
underground layers of sediments and rocks that hold this water are called aquifers.
• We
access groundwater by digging wells or drilling bore wells into these aquifers.
• The
depth of groundwater can vary greatly — it might be just a few meters below the
surface or hundreds of meters deep, depending on the region.
• Although
groundwater is a vital source of water, it is not unlimited.
• Increasing
population and their growing water needs have caused excessive extraction
of groundwater.
• Urbanization
has reduced the natural areas where water can seep into the ground:
1. Less vegetation cover means fewer plants and trees to help water
infiltrate.
2. More concrete surfaces (roads, buildings) prevent water from soaking
into the soil.
• Due
to these factors, the rate of groundwater recharge is reduced, leading to groundwater
depletion.
• To
conserve and replenish groundwater, techniques like:
1. Rainwater harvesting — collecting and storing rainwater for later
use.
2. Recharge pits — specially made pits that help rainwater seep into the
ground.
• These
methods help recharge groundwater, supporting the natural water cycle
and ensuring a sustainable supply of groundwater for future needs.
• Water
scarcity makes life hard, so people find ways to save water.
• In
Ladakh, they make ice stupas—artificial ice cones built in winter.
• These
ice stupas melt slowly in warmer months, providing water for farming and daily
use.
Science
and Society: Ice Stupa
• In
Ladakh, spring streams often dry up because the sun’s heat is not enough to
melt mountain snow quickly.
• During
winter, water from mountain streams is sent through underground pipes and
sprayed into the cold air.
• The
water freezes layer by layer, forming a tall, cone-shaped ice structure called
an ice stupa.
• The
ice stupa melts slowly in spring, supplying water for farming and other needs
throughout summer.
Ice Stupa
7
Answer
• Conduction: The process of heat transfer through direct contact, where particles pass heat to neighboring particles without moving.• Convection: The transfer of heat in liquids and gases by the movement of heated, less dense particles.
• Radiation: The transfer of heat through waves, without needing a medium, as seen in heat from the Sun or a fire.
• Insulator: A material (e.g., wood, glass) that does not allow heat to pass through easily, also called a poor conductor.
• Evaporation: The process where water turns into vapor due to heat, especially from the Sun.
• Condensation: The cooling of water vapor to form liquid droplets, creating clouds.
• Precipitation: The release of water from clouds as rain, snow, or hail.
• Transpiration: The release of water vapor by plants into the atmosphere.
• Aquifer: Underground layers of rock or soil that store groundwater.
• Seepage: The process by which water moves through soil or rock into the ground.
• Bukhari: A traditional Himalayan heater that uses wood or charcoal to warm rooms and cook food.
• Ice Stupa: A cone-shaped ice structure in Ladakh that stores water in winter and releases it in spring.
8
Answer
Heat transfer occurs through three processes: conduction, convection, and radiation. Conduction involves heat moving through solids, like metals, by particle vibrations, making metals ideal for cooking utensils. Convection occurs in liquids and gases, where heated particles move, creating currents like sea and land breezes. Radiation transfers heat without a medium, as seen in the Sun’s heat or warmth from a fire. The water cycle, driven by the Sun’s heat, involves evaporation, condensation, precipitation, and seepage, redistributing and conserving Earth’s water. Groundwater is replenished through seepage, but conservation methods like ice stupas and rainwater harvesting are vital to address scarcity.