NCERT Solutions for Chapter 3 Metals and Non-metals Class 10 Science
Book Solutions
Chapter 3 Metals and Non-metals NCERT Solutions Class 10 Science
By studying the NCERT Solutions for Chapter 3 Metals and Non-metals of Class 10 Science Textbook, students will be able to get good marks in the board examination. This is very important for the students who are studying in CBSE schools. This chapter will provide the students with the basic idea of classification of metals and non-metals and its properties. Also, it will let the students know about the metals oxides, ionic compounds, reactivity series and reactions of metals and non-metals. By studying the NCERT Solutions of this chapter, one could be able to answers the questions related to metals and non-metals which could be asked in the examination.
NCERT Solutions for Chemical Reactions and Equations Class 10 Science
Chapter Name | NCERT Solutions for Chapter 3 Metals and Non-metals |
Subject | Class 10 Science |
Topics covered in the Chapter |
|
Related Readings |
|
These NCERT Solutions of Class 10 NCERT Science Textbook is based on the latest syllabus of CBSE. One could study these questions and answers of metals and non-metals to give their best shot in the examination. One could also take help from the Revision Notes provided of this chapter to study well. These could be found on the links given in this page.
1
Answer
(i) Mercury
(ii) Sodium
(iii) Silver
(iv) Mercury and Lead
2
Answer
Malleable: A substance is said to be malleable if it can be beaten into sheets for example, metals are malleable.
Ductile: A substance is said to be ductile if it can be drawn into wires. for example, metals are ductile.
1
Why is sodium kept immersed in kerosene oil?
Answer
Sodium metal is highly reactive. If it is kept open, it can explosively react with oxygen and moisture present in the air. To prevent this explosive reaction and the after effects, sodium is kept immersed in kerosene oil.
2
Write equations for the reactions of
(i) iron with steam
(ii) calcium and potassium with water
Answer
(i) 3Fe(s) + 4H2O(g) → Fe3O4(aq) + 4H2(g)
(ii) Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g) + Heat
2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) + Heat
3
Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
|
Metal |
Iron (II) sulphate |
Cooper (II) sulphate |
Zinc sulphate |
Silver nitrate |
|
A. |
No reaction |
Displacement |
||
|
B. |
Displacement |
No reaction |
||
|
C. |
No reaction |
No reaction |
No reaction |
Displacement |
|
D. |
No reaction |
No reaction |
No reaction |
No reaction |
(i) Which is the most reactive metal?
(ii) What would you observe if B is added to a solution of copper (II) sulphate ?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
Answer
(i) B is the most reactive metal.
(ii) B will displace copper from copper sulphate solution.
(iii) The decreasing order of reactivity is: B>A>C>D
4
Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute H2SO4
Answer
Hydrogen gas is produced when dilute hydrochloric acid is added to a reactive metal. The chemical reaction between iron and dilute H2SO4 is:
Fe(s) + H2SO4(aq) → FeSO4(aq) + H2(g)
5
What would you observe when zinc is added to a solution of iron (II) sulphate? Write the chemical reaction that takes place.
Answer
When zinc is added to a solution of iron (II) sulphate, it will displace iron from it and light green colour of solution gradually fades away.
Zn(s) + FeSO4(aq) → ZnSO4(aq) + Fe(s)
1
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
Answer
(i) Electron-dot structures for sodium, oxygen and magnesium are:![]()
(ii) Formation of Na2O![]()
Formation of MgO![]()
(iii) Ions present in these compounds are: Na+ and O2-, Mg2+ and O2-
2
Answer
Ionic compounds have strong electrostatic forces of attraction between oppositely charged ions. A considerable amount of energy is required to break these forces of attraction. Thus, ionic compounds they have high melting points.
1
Define the following terms.
(a) Mineral
Answer
(a) Mineral: The free or combined states of elements naturally occurring in the earth's crust are called minerals.
(b) Ore: An ore is a mineral from which a metal can be extracted conveniently and economically.
(c) Gangue: The earthy impurities such as sand, lime stone, rocks etc. associated with minerals are collectively known as gangue or matrix.
2
Name two metals which are found in nature in the free state.
Answer
The metals at the bottom of the reactivity series are mostly found in free state. Two examples are Gold and Platinum.
3
What chemical process is used for obtaining a metal from its oxide?
Answer
A metal can be extracted from its oxide by the process of reduction.
![]()
1
Metallic oxides of zinc, magnesium and copper were heated with the following metals.
|
Metal |
Zinc |
Magnesium |
Copper |
|
Zinc oxide |
- |
- |
- |
|
Magnesium oxide |
- |
- |
- |
|
Copper oxide |
- |
- |
- |
In which cases will you find displacement reactions taking place?
Answer
|
Metal |
Zinc |
Magnesium |
Copper |
|
Zinc oxide |
No reaction |
Displacement |
No reaction |
|
Magnesium oxide |
No reaction |
No reaction |
No reaction |
|
Copper oxide |
Displacement |
Displacement |
No reaction |
Zinc can displace copper from copper oxide.
Zn + CuO → ZnO + Cu
Magnesium can displace zinc and magnesium from zinc oxide and magnesium oxide respectively.
Mg + ZnO → MgO + Zn
Mg + CuO → ZnO + Cu
2
Which metals do not corrode easily?
Answer
Less reactive metals such as silver, gold that are not attacked by air and moisture don't corrode easily.
3
What are alloys?
Answer
Alloys are the homogeneous mixtures of two or more metals or metals and non-metals. For example brass is an alloy of copper and zinc.
1
1. Which of the following pairs will give displacement reactions?
(a) NaCl solution and copper metal
(b) MgCl2 solution and aluminium metal
(c) FeSO4 solution and silver metal
(d) AgNO3 solution and copper metal.
Answer
(d) AgNO3 solution and copper metal.
2
Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) all of the above.
Answer
(c) Applying a coating of zinc
3
An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) calcium
(b) carbon
(c) silicon
(d) iron
Answer
(a) calcium
4
Answer
(c) zinc is more reactive than tin.
5
You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer
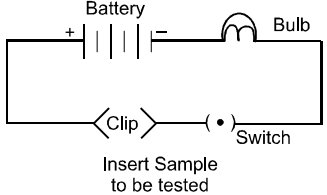
(i) Using the given battery, bulb, wires and switch, set up an electric circuit as shown. Then, insert the samples between clips A and B one by one. If the bulb glows, the sample is a metal. If the bulb does not glow, the sample is a non-metal. Likewise, beat the samples using the given hammer one by one. If a sample can be beaten into a thin sheet, it is a metal otherwise it is a non-metal.
(ii) These tests are useful in distinguishing between metals and non-metals on the basis of their electrical conductivity and malleability. Metals are good conductor of electricity whereas non-metals are bad. Metals are malleable whereas non-metals are non-malleable.
6
Answer
Those oxides that behave as both acidic and basic oxides are called amphoteric oxides.Examples: aluminium oxide (Al2O3), zinc oxide (ZnO)
7
Answer
Iron and Magnesium will displace hydrogen from dilute acids as they more reactive then hydrogen.8
Answer
In the electrolytic refining of a metal M:
Anode → Impure metal M
Cathode → Thin strip of pure metal M
Electrolyte → Salt Solution of the metal M
9
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
Answer
(a) (i) No action on dry litmus paper.
(ii) The colour of litmus paper will turn red. It happens because sulphur is a non-metal and the oxides of non-metal are acidic in nature.
(b) S(s) + O2(g) → S O2(g)
10
Answer
Two ways to prevent the rusting of iron are:
→ Oiling, greasing, or painting: By applying oil, grease, or paint, the surface becomes water proof and the moisture and oxygen present in the air cannot come into direct contact with iron. Hence, rusting is prevented.
→ Galvanisation: An iron article is coated with a layer of zinc metal, which prevents the iron to come in contact with oxygen and moisture. Hence, rusting is prevented.
11
Answer
When non-metals are combined with oxygen they form two types of oxides.
(i) Acidic oxides such as NO2, SO2
(ii) Neutral oxides such as NO, CO etc.
12
Answer
(a) Platinum, gold, and silver are used to make jewellery because they are very lustrous. Also, they are very less reactive and do not corrode easily. These metals are highly malleable and ductile.
(b) Sodium, potassium, and lithium are highlyreactive metals and react very vigorously with air as well as water. Therefore, they are kept immersed in kerosene oil in order to prevent their contact with air and moisture.
(c) Though aluminium is a highly reactive metal, it is resistant to corrosion. This is because aluminium reacts with oxygen present in air to form a thin layer of aluminium oxide. This oxide layer is very stable and prevents further reaction of aluminium with oxygen. Also, it is light in weight and a good conductor of heat. Hence, it is used to make cooking utensils.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction because metals can be easily extracted from their oxides rather than from their carbonates and sulphides.
13
Answer
Copper reacts with moist carbon dioxide in air to form copper carbonate and as a result, copper vessel loses its shiny brown surface forming a green layer of copper carbonate. The citric acid present in the lemon or tamarind neutralises the basis copper carbonate and dissolves the layer. That is why, tarnished copper vessels are cleaned with lemon or tamarind juice to give the surface of the copper vessel its characteristic lustre.
14
Answer
Difference between Metals and Non-metals on the basis of their chemical properties:|
Metal |
Non-metal |
|
Metals are electropositive. |
Non-metals are electronegative. |
|
Oxides of metal are basic in nature. |
Oxides of non-metals are acidic in nature. |
|
Metals displace hydrogen from dilute acids. |
They can't replace hydrogen from dilute acids. |
|
Metals form chlorides which are electrovalent or ionic compounds. |
Non-metals form chlorides which are covalent compounds. |
|
They react with water to form oxides and hydroxides. Some metals react with cold water, some with hot water, and some with steam. |
They do not react with water. |
15
Answer
The solution he had used was Aqua regia. Aqua regia is Latin word which means ‘Royal Water’. It is the mixture of concentrated Hydrochloric acid(HCl) and concentrated nitric acid(HNO3) in the ratio of 3:1. It is capable of dissolving metals like Gold and Platinum. Since the outer layer of the gold bangles is dissolved in aqua regia so their weight was reduced drastically.16
Answer
Copper does not react with cold water, hot water, or steam. However, iron reacts with steam. If the hot water tanks are made of steel (an alloy of iron), then iron would react vigorously with the steam formed from hot water.
3Fe + 4H2O → Fe3O4 + H2O
That is why copper is used to make hot water tanks, and not steel.