Coordination Compounds
Book Solutions1
Answer
( a ) A metal shows two kinds of valencies viz primary valency and secondary valency.Negative ionssatisfy primary valenciesand secondaryvalencies are filled by bothneutral ions and negative ions.( b ) A metal ion has a fixed amount of secondary valencies about the central atom. These valenciesalso orient themselves in a particular direction in the space provided to the definite geometry of the coordination compound.
( c ) Secondary valencies cannot be ionized , while primary valencies can usually be ionized.
2
Answer
3
Answer
4
5
Specify the oxidation numbers of the metals in the following coordination entities:
(i) [Co(H2O)(CN)(en)2]2+
(ii) [CoBr2(en)2]+
(iii) [PtCl4]2−
(iv) K3[Fe(CN)6]
(v) [Cr(NH3)3Cl3]
Answer
6
(i) Tetrahydroxozincate(II)
(ii) Potassium tetrachloridopalladate(II)
(iii) Diamminedichloridoplatinum(II)
(iv) Potassium tetracyanonickelate(II)
(v) Pentaamminenitrito-O-cobalt(III)
(vi) Hexaamminecobalt(III) sulphate
(vii) Potassium tri(oxalato)chromate(III)
(viii) Hexaammineplatinum(IV)
(ix) Tetrabromidocuprate(II)
(x) Pentaamminenitrito-N-cobalt(III)
Answer
(i) [Zn(OH)4]2−
(ii) K2[PdCl4]
(iii) [Pt(NH3)2Cl2]
(iv) K2[Ni(CN)4]
(v) [Co(ONO) (NH3)5]2+
(vi) [Co(NH3)6]2 (SO4)3
(vii) K3[Cr(C2O4)3]
(viii) [Pt(NH3)6]4+
(ix) [Cu(Br)4]2−
(x) [Co[NO2)(NH3)5]2+
7
Using IUPAC norms write the systematic names of the following:
(i) [Co(NH3)6]Cl3
(ii) [Pt(NH3)2Cl(NH2CH3)]Cl
(iii) [Ti(H2O)6]3+
(iv) [Co(NH3)4Cl(NO2)]Cl
(v) [Mn(H2O)6]2+
(vi) [NiCl4]2−
(vii) [Ni(NH3)6]Cl2
(viii) [Co(en)3]3+
(ix) [Ni(CO)4]
Answer
(i) Hexaammine cobalt(III) chloride
(ii) Diammine chlorido (methylamine) platinum(II) chloride
(iii) Hexaqua titanium(III) ion
(iv) Tetraammine chlorido nitrito-N-Cobalt(III) chloride
(v) Hexaqua manganese(II) ion
(vi) Tetrachlorido nickelate(II) ion
(vii) Hexaammine nickel(II) chloride
(viii) Tris(ethane-1, 2-diammine) cobalt(III) ion
(ix) Tetracarbonyl nickel(0)
8
Answer
9
How many geometrical isomers are possible in the following coordination entities?
(i) [Cr(C2O4)3]3− (ii) [Co(NH3)3Cl3]
Answer
10
Draw the structures of optical isomers of:
(i) [Cr(C2O4)3]3−
(ii) [PtCl2(en)2]2+
(iii) [Cr(NH3)2Cl2(en)]+
Answer
11
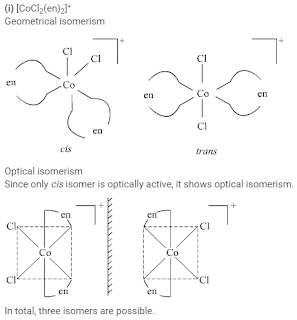
Draw all the isomers (geometrical and optical) of:
(i) [CoCl2(en)2]+
(ii) [Co(NH3)Cl(en)2]2+
(iii) [Co(NH3)2Cl2(en)]+
Answer
12
Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how many of these will exhibit optical isomers?
Answer
13
Aqueous copper sulphate solution (blue in colour) gives:
(i) a green precipitate with aqueous potassium fluoride, and
(ii) a bright green solution with aqueous potassium chloride
Explain these experimental results.
Answer
14
Answer
15
Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4−
(ii) [FeF6]3−
(iii) [Co(C2O4)3]3−
(iv) [CoF6]3−
Answer
16
Answer
17
Answer
A spectrochemical series is the arrangement of common ligands in the increasing order of their crystal-field splitting energy (CFSE) values. The ligands present on the R.H.S of the series are strong field ligands while that on the L.H.S are weak field ligands. Also, strong field ligands cause higher splitting in the d orbitals than weak field ligands.
I− < Br− < S2− < SCN− < Cl−< N3 < F− < OH− < C2O42− ∼ H2O < NCS− ∼ H− < CN− < NH3 < en ∼ SO32− < NO2− < phen < CO
18
Answer
The degenerate d-orbitals (in a spherical field environment) split into two levels i.e., eg and t2g in the presence of ligands. The splitting of the degenerate levels due to the presence of ligands is called the crystal-field splitting while the energy difference between the two levels (eg and t2g) is called the crystal-field splitting energy. It is denoted by Δo.
After the orbitals have split, the filling of the electrons takes place. After 1 electron (each) has been filled in the three t2gorbitals, the filling of the fourth electron takes place in two ways. It can enter the eg orbital (giving rise to t2g3 eg1 like electronic configuration) or the pairing of the electrons can take place in the t2g orbitals (giving rise to t2g4 eg0 like electronic configuration). If the Δo value of a ligand is less than the pairing energy (P), then the electrons enter the egorbital. On the other hand, if the Δo value of a ligand is more than the pairing energy (P), then the electrons enter the t2gorbital.
19
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Answer
20
Answer
In [Ni(H2O)6]2+, H2O is a weak field ligand. Therefore, there are unpaired electrons in Ni2+. In this complex, the d electrons from the lower energy level can be excited to the higher energy level i.e., the possibility of d−d transition is present. Hence, Ni(H2O)6]2+ is coloured.
In [Ni(CN)4]2−, the electrons are all paired as CN- is a strong field ligand. Therefore, d-d transition is not possible in [Ni(CN)4]2−. Hence, it is colourless.
21
Answer
The colour of a particular coordination compound depends on the magnitude of the crystal-field splitting energy, Δ. This CFSE in turn depends on the nature of the ligand. In case of [Fe(CN)6]4− and [Fe(H2O)6]2+, the colour differs because there is a difference in the CFSE. Now, CN− is a strong field ligand having a higher CFSE value as compared to the CFSE value of water. This means that the absorption of energy for the intra d-d transition also differs. Hence, the transmitted colour also differs.22
Answer
The metal-carbon bonds in metal carbonyls have both σ and π characters. A σ bond is formed when the carbonyl carbon donates a lone pair of electrons to the vacant orbital of the metal. A π bond is formed by the donation of a pair of electrons from the filled metal d orbital into the vacant anti-bonding π* orbital (also known as back bonding of the carbonyl group). The σ bond strengthens the π bond and vice-versa. Thus, a synergic effect is created due to this metal-ligand bonding. This synergic effect strengthens the bond between CO and the metal.23
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complexes:
(i) K3[Co(C2O4)3]
(ii) cis-[Cr(en)2Cl2]Cl
(iii) (NH4)2[CoF4]
(iv) [Mn(H2O)6]SO4
Answer
(i) K3[Co(C2O4)3]
The central metal ion is Co.
Its coordination number is 6.
The oxidation state can be given as:
x − 6 = −3
x = + 3
The d orbital occupation for Co3+ is t2g6eg0.
(ii) cis-[Cr(en)2Cl2]Cl
The central metal ion is Cr.
The coordination number is 6.
The oxidation state can be given as:
x + 2(0) + 2(−1) = +1
x − 2 = +1
x = +3
The d orbital occupation for Cr3+ is t2g3.
(iii) (NH4)2[CoF4]
The central metal ion is Co.
The coordination number is 4.
The oxidation state can be given as:
x − 4 = −2
x = + 2
The d orbital occupation for Co2+ is eg4 t2g3.
(iv) [Mn(H2O)6]SO4
The central metal ion is Mn.
The coordination number is 6.
The oxidation state can be given as:
x + 0 = +2
x = +2
The d orbital occupation for Mn is t2g3 eg2.
24
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
(i) K[Cr(H2O)2(C2O4)2].3H2O
(ii) [Co(NH3)5Cl]Cl2
(iii) CrCl3(py)3
(iv) Cs[FeCl4]
(v) K4[Mn(CN)6]
Answer
(i) Potassium diaqua dioxalato chromate (III) trihydrate.
Oxidation state of chromium = 3
Electronic configuration: 3d3: t2g3
Coordination number = 6
Shape: octahedral
magnetic moment = ∼ 4BM
(ii) [Co(NH3)5Cl]Cl2
IUPAC name: Pentaammine chloride cobalt(III) chloride
Oxidation state of Co = +3
Coordination number = 6
Shape: octahedral.
Electronic configuration: d6: t2g6.
magnetic moment = 0
(iii) CrCl3(py)3
IUPAC name: Trichlorido tripyridine chromium (III)
Oxidation state of chromium = +3
Electronic configuration for d3 = t2g3
Coordination number = 6
Shape: octahedral.
(iv) Cs[FeCl4]
IUPAC name: Caesium tetrachloroferrate (III)
Oxidation state of Fe = +3
Electronic configuration of d6 = eg2t2g3
Coordination number = 4
Shape: tetrahedral
Stereochemistry: optically inactive
n = 5
magnetic moment = ∼ 6 BM
(v) K4[Mn(CN)6]
Potassium hexacyanomanganate(II)
Oxidation state of manganese = +2
Electronic configuration: d5+: t2g5
Coordination number = 6
Shape: octahedral.
Streochemistry: optically inactive
n = 1
magnetic moment = 1.732 BM
25
Answer
Stability of a coordination compound in a solution is the degree/level of association among the species involved in a state of equilibrium.
Stability can also be written quantitatively in terms of formation constant or stability constant.
( 1 ) Charge on the central metal ion – bigger the charge, more stable is the complex.
( 2 ) Nature of ligand – chelating ligand produces a more stable complex.
( 3 ) The basic strength of ligand- more basic a ligand, more stable its complex.
26
Answer
When a ligand attaches to the metal ion in a manner that forms a ring, then the metal- ligand association is found to be more stable. In other words, we can say that complexes containing chelate rings are more stable than complexes without rings. This is known as the chelate effect.27
Discuss briefly giving an example in each case the role of coordination compounds in:
(i) biological system
(ii) medicinal chemistry
(iii) analytical chemistry
(iv) extraction/metallurgy of metals
Answer
( a ) Role in biological systems:In the body of animals, there are several very important coordination compounds e.g. hemoglobin is a coordination compound of iron.
In plants, the chlorophyll pigment is a coordination compound of magnesium.
( b ) Role in medicinal chemistry:
So many coordinate compounds are used for curing purposes. For e.g., a coordination compound of platinum, cis-platin is used for checking the growth of tumors.
( c ) Role in analytical chemistry:
Determination of hardness of water.
( d ) Role in metallurgy or extraction:
During metal extraction from ores, complexes are formed. For e.g. gold combines with cyanide ions in an aqueous solution. Gold is then extracted from this complex using zinc.
28
How many ions are produced from the complex Co(NH3)6Cl2 in solution?
(i) 6
(ii) 4
(iii) 3
(iv) 2
Answer
(iii) The given complex can be written as [Co(NH3)6]Cl2.
Thus, [Co(NH3)6]+ along with two Cl− ions are produced.
29
Amongst the following ions which one has the highest magnetic moment value?
(i) [Cr(H2O)6]3+
(ii) [Fe(H2O)6]2+
(iii) [Zn(H2O)6]2+
Answer
30
The oxidation number of cobalt in K[Co(CO)4] is
(i) +1
(ii) +3
(iii) −1
(iv) −3
Answer
We know that CO is a neutral ligand and K carries a charge of +1.
Therefore, the complex can be written as K+[Co(CO)4]−. Therefore, the oxidation number of Co in the given complex is −1. Hence, option (iii) is correct.
31
Amongst the following, the most stable complex is
(i) [Fe(H2O)6]3+
(ii) [Fe(NH3)6]3+
(iii) [Fe(C2O4)3]3−
(iv) [FeCl6]3−
Answer
32
What will be the correct order for the wavelengths of absorption in the visible region for the following:
[Ni(NO2)6]4−, [Ni(NH3)6]2+, [Ni(H2O)6]2+
Answer
